GSK losing a bid to keep experts out of an upcoming Zantac trial.

A California judge on Thursday denied GSK Plc's bid to keep expert testimony linking its discontinued heartburn drug Zantac to cancer out of an upcoming trial, a setback for the British drugmaker facing lawsuits over the medicine in courts across the United States.
Analysts said that while Alameda County Superior Court Judge Evelio Grillo's ruling was not surprising, the litigation will likely weigh on the drugmaker's share price until the trial, scheduled to begin July 24.
First approved in 1983, Zantac became the world's best selling medicine in 1988 and one of the first-ever drugs to top $1 billion in annual sales.
The trial will be the first test of how Zantac cancer claims will fare before a jury. The plaintiff, California resident James Goetz, says he developed bladder cancer from taking the drug.
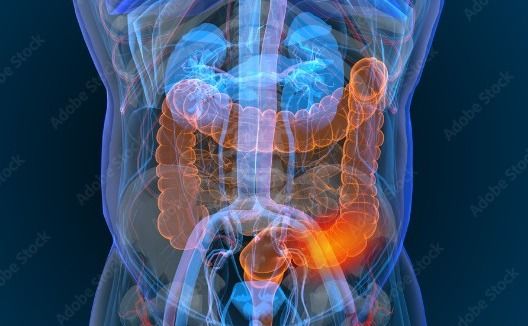
Zantac is a brand name for the drug ranitidine, which is an over-the-counter and prescription medication used to treat heartburn and other gastric disorders. In September 2019, the FDA announced that it had found low levels of a probable human carcinogen called N-nitrosodimethylamine (NDMA) in some ranitidine products, including Zantac. This led to a series of voluntary recalls and the eventual removal of Zantac and other ranitidine products from the market.
GlaxoSmithKline (GSK) is one of the companies that manufactured and distributed Zantac. Following the recalls, numerous lawsuits were filed against GSK and other pharmaceutical companies, claiming that they failed to warn consumers about the risks associated with the drug and that the use of Zantac caused various forms of cancer.
In such legal proceedings, it's common for both plaintiffs and defendants to present expert witnesses to provide testimony and opinions on complex scientific or technical matters related to the case. It's possible that GSK sought to exclude certain experts from testifying in an upcoming trial related to Zantac, but without more up-to-date information, I cannot provide specific details on the outcome of that bid.
The FDA in 2020 pulled all remaining brand name Zantac and generic versions off the market, triggering a wave of lawsuits.
Analysts said it was not surprising that Grillo ruled differently from the federal court because California's courts are known to be friendlier to plaintiffs.
Citi analysts said the likely magnitude of any settlement for GSK is "likely very modest," at less than $5 billion, and noted that the statute of limitations would somewhat restrict a mushrooming of cases.
Get Cash From Your Case Now
From the Leader in Pre-settlement Funding