FDA Recalls Three Eye Drop Brands Due to Infections, Vision Loss, and Death.

It's crucial for us to be aware of and share vital information that affects our families health and well-being. Today, we bring your attention to a serious issue: the Food and Drug Administration (FDA) has recently issued an alarming recall of three eye drop brands.
One of these brands has been linked to grave consequences, including infections, vision loss, and even death. In this blog post, we will delve into the details of this recall and provide you with essential tips on how to keep your loved ones safe.

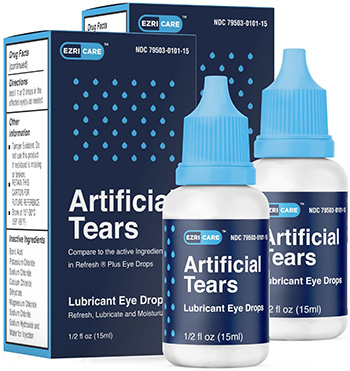
- Artificial Tears Lubricant Eye Drops distributed by EzriCare, LLC and DELSAM Pharma. On Feb. 2, the FDA issued a warning not to use EzriCare Artificial Tears because of potential bacterial contamination. The over-the-counter product was associated with severe eye infections in 55 patients, including one death. The infections were caused by a drug-resistant bacteria, Pseudomonas aeruginosa.
- Brimonidine Tartrate Ophthalmic Solution, 0.15%. Apotex Corp. initiated a voluntary recall for six lots of Brimonidine Tartrate Ophthalmic Solution on March 1 due to cracks in the caps. The prescription drops are used for patients with open-angle glaucoma or ocular hypertension. No infections have been associated with the product.
- Purely Soothing 15% MSM Drops. The FDA announced on March 3 that Pharmedica USA was voluntarily recalling two lots of Purely Soothing Drops. This over-the-counter product is being recalled due to non-sterility. There have not been reports of illness or infection related to the product.
In addition to drops, the FDA has also recalled Global Pharma Healthcare Artificial Eye Ointment due to possible bacterial contamination. No infections have been associated with this over-the-counter product.
“If you are using any of these specific products, stop,” said Gary D. Novack, a professor at UC Davis Health. Novack is a clinical pharmacologist with decades of experience in ophthalmic product development.
The UC Davis Eye Center has not seen any cases linked to the EzriCare drops, but infections have been reported in 12 states, including California.
“Using contaminated artificial tears increases the risk of eye infections that could result in blindness or serious illness,” Novack said.
If you or a family member have used any of the recalled eye drops, it's important to be aware of the following symptoms that could indicate an infection or vision loss:
- Redness and swelling of the eye
- Pain or discomfort
- Sensitivity to light
- Blurry vision
- Discharge from the eye
- Floaters or flashing lights in your field of vision
If any of these symptoms are present, seek immediate medical attention. Early treatment is critical in preventing severe complications and preserving vision.
The FDA's recent recall of three eye drop brands is a reminder of the importance of staying vigilant about our families' health and well-being. We hope this blog post has provided you with valuable information to protect your loved ones from the potentially devastating effects of using these recalled products.