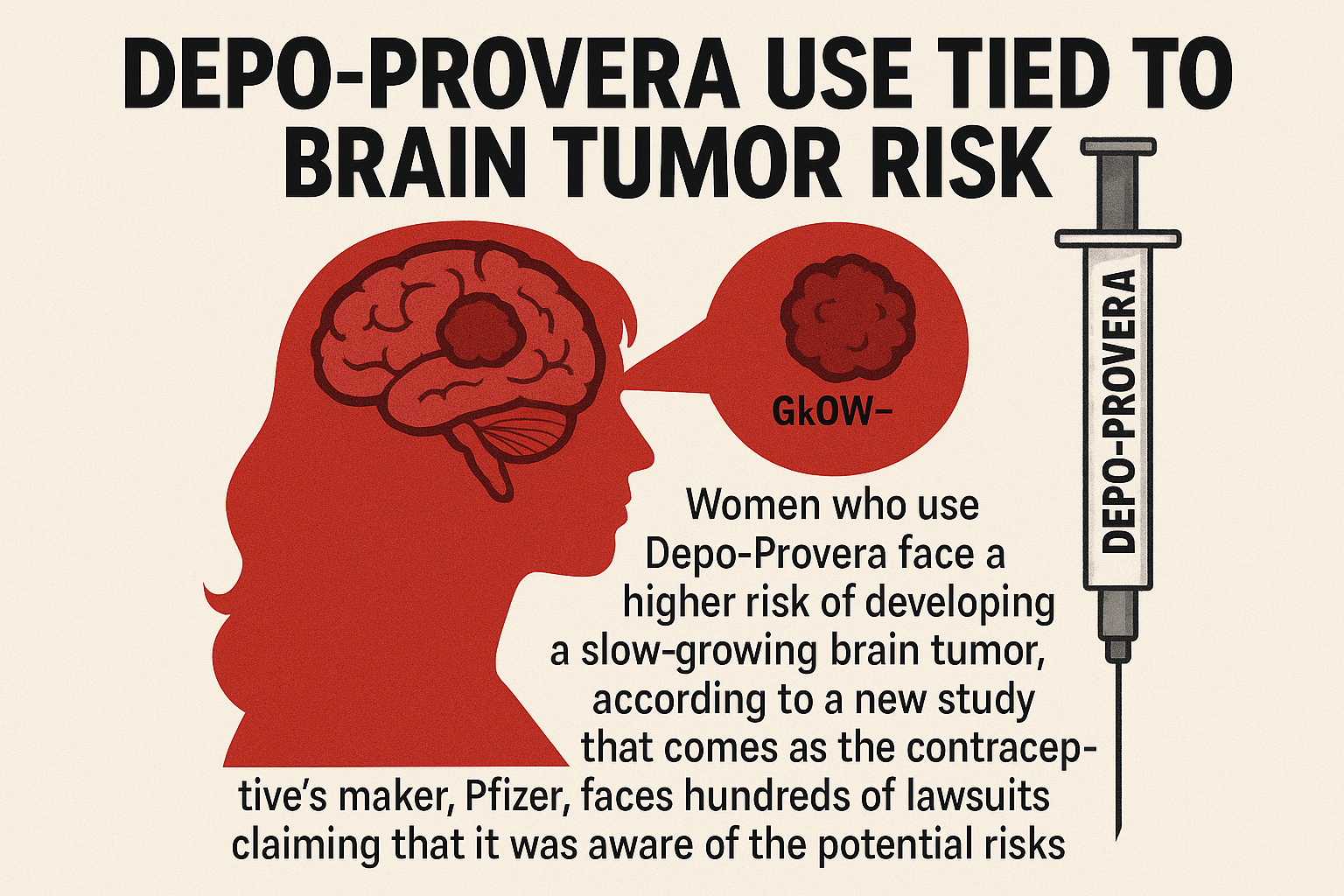
Pfizer’s Depo-Provera—has been linked to BRAIN TUMORS in a massive new study.

PFIZER’S BIRTH CONTROL CRISIS: THE DRUG THEY SAID WAS SAFE
October 17, 2025
“Corporations have neither bodies to be punished, nor souls to be condemned.” – Thurlow, 1783
A billion-dollar drug empire is quietly facing collapse. Pfizer—the multinational hailed for vaccines and ‘safe’ contraceptives—is now defending itself against more than a thousand women who say its Depo-Provera birth control shot gave them brain tumors. For decades, doctors brushed off complaints of migraines, vision loss, and seizures as “rare side effects.” But new clinical data and courtroom evidence suggest something darker: Pfizer may have known more than it ever let on.
The truth isn’t what they sold you.

THE TIMELINE
1954 (SYNTHESIS): Medroxyprogesterone acetate is synthesized to treat gynecological disorders like fibroids and endometriosis.
1960s–1991: Despite concerns over cancer risk, Depo-Provera is used as a contraceptive overseas. The FDA repeatedly rejects its use in the U.S. for fear of tumors.
1992 (APPROVAL): After pressure and new studies, Pfizer finally wins FDA approval. No tumor warning appears on the label.
2004: A “black box” label is added—not for tumors, but bone density loss.
2023 (THE FRENCH WARNING): A major BMJ study finds women using Depo-Provera for more than a year have a 5.6x higher risk of brain meningioma. It triggers alarm in Europe—yet Pfizer’s label remains unchanged in the U.S.
2024–2025 (THE LEGAL EXPLOSION): Women begin filing lawsuits. By October 2025, over 1,200 cases are consolidated in federal multidistrict litigation (MDL) under Judge Casey Rodgers in Florida.
SEPTEMBER 2025 (THE JAMA STUDY): Researchers at the Cleveland Clinic and Case Western publish an analysis of 10 million patient records—confirming women using Depo-Provera face a 2.43x higher risk of meningioma, especially those starting after age 31 or using for four-plus years.
OCTOBER 2025: Pfizer claims the FDA prevented warning updates. Plaintiffs counter: Pfizer told only half the story.
CLAIM ONE: PFIZER KNEW ABOUT THE RISK
The lie that started it all.
Public Narrative: Depo-Provera was a well-researched, safe hormonal contraceptive that helped millions of women manage family planning. Pfizer insists it always disclosed risks appropriately and that no evidence ever justified a brain tumor warning.
Findings: Medical literature dating back to 1983 linked synthetic progesterones—like those in Depo-Provera—to meningioma growth. Plaintiffs’ filings allege Pfizer had access to these studies long before FDA approval but chose to pursue commercial clearance rather than further safety studies. The company itself admitted in court filings that it discovered a “potential causal link” as early as 2023—decades after the earliest red flags.
Analysis: Pfizer’s defense turns regulation into a shield. By citing “federal preemption,” it claims its hands were tied. But documents suggest it knowingly starved the FDA of full data, creating the very conditions that blocked a warning. That’s not oversight—it’s orchestration.
CLAIM TWO: THE FDA WAS MISLED
When regulators rely on half-truths, public safety collapses.
Public Narrative: Pfizer says it submitted all relevant safety information and even petitioned the FDA for a warning label, but the agency declined, finding "insufficient evidence."
Findings: Plaintiffs allege Pfizer cherry-picked data to make its 2023 submission appear weak, ensuring denial. According to court filings, Pfizer’s internal pharmacovigilance unit documented “signal detection” of progesterone-linked tumor cases years before disclosure but never escalated them to the FDA. When it finally did, it downplayed severity in statistical models.
Analysis: The FDA denial now looks less like an error—and more like the outcome Pfizer engineered. The revolving door between Big Pharma and regulators meant watchdogs became lapdogs. Every informed denial was a victory for shareholders, not science.
CLAIM THREE: THE HUMAN COST IS DEVASTATING
Behind every statistic is a surgery scar.
Public Narrative: Meningiomas are typically benign, treatable brain tumors. Most patients recover. The risk remains “low.”
Findings: Plaintiffs’ stories tell another tale. Louisiana’s Robin Phillip lost vision in her left eye after an intracranial meningioma compressed her optic nerve. Others report seizures, cognitive impairment, or permanent disability. Ellen Relkin, lead plaintiffs’ counsel, confirms “many underwent brain surgery and radiation; their lives are permanently changed.”
Analysis: When one in 10,000 women suffers a tumor that could have been prevented by a simple label change, the moral calculus changes. These aren’t isolated anecdotes—they’re epidemiological red flags. Each dismissed complaint was another brick in Pfizer’s legal dam, now cracking under the weight of evidence and outrage.
CLAIM FOUR: PFIZER’S LEGAL STRATEGY — DENY, DELAY, DEFLECT
Preemption isn’t protection; it’s evasion.
Public Narrative: Because the FDA denied Pfizer’s request to update Depo-Provera’s label, state “failure-to-warn” lawsuits can’t proceed—a key “preemption” argument meant to end litigation.
Findings: Internal memos show Pfizer anticipated this defense years in advance. According to the September 30 preemption hearing summaries, plaintiffs presented evidence that Pfizer “withheld critical safety reports” and “selectively diluted safety data” before seeking FDA review. Legal observers note this could nullify preemption entirely.
Analysis: Pfizer is fighting the case not on facts—but on technicalities. Like Johnson & Johnson in its talc litigation, it’s betting that process can outlast pain. Yet even Wall Street is nervous: Pfizer’s Q4 filings hint at potential “material financial exposure” if the MDL proceeds to trial.
CLAIM FIVE: PUBLIC HEALTH BETRAYED
The system trusted to protect women has failed them.
Public Narrative: The FDA monitors prescription drug safety proactively, and datasets are continuously updated based on emerging science.
Findings: Instead, the system relies on voluntary manufacturer reporting and self-audited adverse event data. No independent oversight ensured Pfizer’s reports were complete. A quarter of sexually active American women have used Depo-Provera at some point; none were ever warned about tumor risk.
Analysis: This scandal isn’t just about Pfizer—it’s about the architecture of pharmaceutical oversight that rewards plausible deniability. When bureaucracy replaces accountability, harm becomes part of the business model.
EPILOGUE
The Cleveland Clinic findings aren’t the end—they’re the beginning of an epochal reckoning for women’s health. The lawsuits, now more than 1,200 strong, could force disclosure of internal Pfizer communications long sealed from public view. If those messages confirm what early evidence suggests, this case will redefine corporate accountability in medicine.
Because when science warns and silence follows—it’s not ignorance. It’s intent.